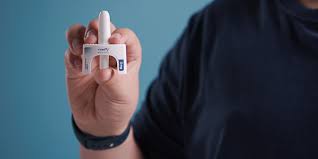
In a groundbreaking development, the U.S. Food and Drug Administration (FDA) has approved the first-ever nasal spray for the treatment of anaphylaxis. This approval represents a significant advancement in the management of severe allergic reactions, offering a needle-free alternative to traditional epinephrine injections. This article delves into the details of this new treatment, its implications for patients and healthcare providers, and the broader impact on allergy
Understanding Anaphylaxis
What is Anaphylaxis?
Anaphylaxis is a severe, potentially life-threatening allergic reaction that can occur rapidly after exposure to an allergen. Common triggers include certain foods, insect stings, medications, and latex. Symptoms of anaphylaxis can include difficulty breathing, swelling of the throat, a rapid drop in blood pressure, and loss of consciousness. Immediate treatment is crucial to prevent serious complications or
Traditional Treatment Approaches
Traditionally, the primary treatment for anaphylaxis involves the administration of epinephrine, usually delivered via an auto-injector. Epinephrine helps counteract the severe allergic reaction by constricting blood vessels, relaxing airway muscles, and reducing swelling. While effective, the use of epinephrine auto-injectors can be challenging for some patients, particularly in high-stress situations or for those who have difficulty using the device correctly.
Table of Contents
The New Nasal Spray: Overview and Features
FDA Approval and Product Details
The FDA’s recent approval of the nasal spray for anaphylaxis marks a significant milestone in allergy treatment. This new medication, developed by [Manufacturer’s Name], offers a needle-free option for delivering epinephrine. The nasal spray is designed to be easy to use and effective in rapidly delivering the medication during an anaphylactic episode.
The nasal spray works by administering epinephrine through the nasal mucosa, which allows for rapid absorption into the bloodstream. The device is designed to be user-friendly, with a straightforward mechanism for administration that does not require the precision of a needle.
How It Works
The nasal spray delivers a pre-measured dose of epinephrine through the nasal passages. The epinephrine is absorbed through the mucous membranes in the nose, which provides a rapid onset of action. This method of delivery offers several advantages, including ease of use and reduced anxiety associated with needle-based treatments.
Implications for Patients and Healthcare Providers
Advantages for Patients
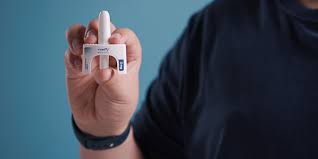
The approval of the nasal spray offers several benefits for patients:
- Ease of Use: The nasal spray is designed to be more user-friendly than traditional auto-injectors. This can be particularly advantageous for individuals who have difficulty using a needle-based device or who experience anxiety related to injections.
- Rapid Action: The nasal spray provides a quick delivery of epinephrine, which is crucial in managing anaphylaxis. The absorption through the nasal mucosa allows for rapid onset of the medication.
- Reduced Discomfort: For those who are needle-averse, the nasal spray offers a less invasive alternative, reducing the discomfort and stress associated with injections.
Considerations for Healthcare Providers
Healthcare providers will need to adapt to the availability of this new treatment option. This includes:
- Patient Education: Educating patients about the proper use of the nasal spray will be essential. Providers will need to ensure that patients understand how to use the device effectively and recognize the signs of anaphylaxis.
- Integration into Treatment Plans: The nasal spray may become part of the standard treatment protocol for anaphylaxis. Providers will need to consider how this option fits into existing treatment plans and guidelines.
- Monitoring and Follow-Up: Continued monitoring of patients who use the nasal spray will be important to assess its effectiveness and address any potential issues or concerns.
Safety and Efficacy
Clinical Trials and Research
The nasal spray underwent rigorous testing in clinical trials to ensure its safety and efficacy. These trials evaluated the drug’s ability to effectively manage anaphylaxis, as well as its safety profile. Results indicated that the nasal spray is a viable alternative to traditional epinephrine auto-injectors, with comparable effectiveness in treating severe allergic reactions.
Potential Side Effects
As with any medication, the nasal spray may have potential side effects. Common side effects of epinephrine can include headache, dizziness, palpitations, and nasal irritation. It is important for patients to be aware of these potential effects and to discuss them with their healthcare provider.
Broader Impact on Allergy Management
Changing the Landscape of Anaphylaxis Treatment
The approval of the nasal spray represents a significant shift in the management of anaphylaxis. By providing a needle-free alternative, it offers greater flexibility and convenience for patients. This development may also lead to increased adherence to treatment and improved outcomes for individuals at risk of severe allergic reactions.
Future Developments
The success of the nasal spray may pave the way for further innovations in allergy treatment. Researchers and pharmaceutical companies may explore additional needle-free delivery systems or improvements in existing treatments. The focus will likely remain on enhancing patient experience and ensuring effective management of severe allergic reactions.
Patient Stories and Testimonials
Real-Life Experiences
Many patients have shared positive experiences with the nasal spray, highlighting its ease of use and effectiveness. For individuals who have struggled with traditional auto-injectors, the nasal spray offers a welcome alternative. Personal testimonials often emphasize the reduced anxiety associated with the needle-free approach and the confidence gained from having a reliable treatment option.
Expert Opinions
Allergists and immunologists have expressed optimism about the nasal spray, noting its potential to improve patient outcomes and simplify anaphylaxis management. Experts emphasize the importance of continued patient education and adherence to treatment protocols to maximize the benefits of this new option.
Conclusion
The FDA’s approval of the nasal spray for anaphylaxis represents a significant advancement in the management of severe allergic reactions. By offering a needle-free alternative to traditional epinephrine injections, the nasal spray provides a user-friendly and effective option for patients at risk of anaphylaxis. As healthcare providers integrate this new treatment into their practices, it is expected to enhance patient care and contribute to improved outcomes in allergy management. The nasal spray’s approval marks a milestone in the ongoing efforts to address the challenges of anaphylaxis and improve the quality of life for those affected by severe allergic reactions.







