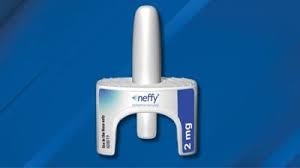
The U.S. Food and Drug Administration (FDA) has recently approved a groundbreaking nasal spray designed to treat severe allergic reactions, marking a significant advancement in allergy treatment. This novel medication offers a new option for individuals who experience life-threatening nasal spray2024allergic reactions, commonly known as anaphylaxis. The approval of this nasal spray represents a major step forward in allergy management and emergency care, providing a more accessible and user-friendly alternative to existing treatments.
The Need for New Treatmentsnasal spray2024
It can lead to symptoms such as difficulty breathing, swelling, hives, and a drop in blood pressure, which can be fatal if not treated promptly.
**1. *Current Treatments:*
- Epinephrine Injections: The standard treatment for anaphylaxis is epinephrine, which is administered via injection. Epinephrine works by constricting blood vessels, relaxing the muscles in the airways, and reducing the release of inflammatory chemicals. These injections are typically delivered using auto-injectors, such as EpiPen, which are designed for emergency use.nasal spray2024
- Limitations of Injections: While epinephrine injections are highly effective, they require proper training for use, and some individuals may find them challenging to administer during a crisis. Additionally, auto-injectors can be expensive and may not be readily available in all situations.
**2. *The Need for a Nasal Spray:*
- Accessibility and Ease of Use: A nasal spray offers a potentially easier and more accessible method for administering treatment during an allergic reaction. Nasal sprays are simpler to use and do not require the precision of an injection, which can be critical in emergency situations.
- Improved Patient Compliance: For individuals with severe allergies, havingnasal spray2024 an additional option like a nasal spray could improve adherence to treatment plans and provide peace of mind knowing that a treatment is readily available.
Approval of the Nasal Spray
The FDA’s approval of the nasal spray marks a significant milestone in the field of allergy treatment. The nasal spray, which contains a form of epinephrine, is designed to be used in emergency situations to treat anaphylaxis.
**1. *Product Details:*
- Formulation: The nasal spray contains epinephrine, the same active ingredient found in traditional auto-injectors. It is formulated for rapid absorption through thenasal spray2024 nasal mucosa, allowing for quick action in emergency situations.
- Administration: The nasal spray is designed for ease of use, with a straightforward mechanism for administration. Users simply insert the nozzle into one of their nostrils and press to release the medication. This simplicity is intended to make it more accessible, especially in high-stress situations.
- Dosage and Effectiveness: Clinical trials have demonstrated that the nasal spray is effective in delivering epinephrine and managing symptoms of anaphylaxis. The dosage is carefully calibrated to match the effectiveness of traditional injections, ensuring that it provides the necessary therapeutic effectnasal spray2024.
**2. *FDA Approval Process:*
- Clinical Trials: The nasal spray underwent rigorous clinical trials to establish its safety and efficacy. These trials involved evaluating the product in various emergency scenarios and assessing its performance compared to traditional treatments.
- Regulatory Review: The FDA’s approval process involved a comprehensive review of clinical data, safety information, and manufacturing practices. The agency’s approval signifies that the nasal spray meets the necessary standards for effectiveness and safety.
Impact on Allergy Management
Table of Contents
The introduction of the nasal spray is expected to have a significant impact on the management of severe allergic reactions and overall allergy care.
**1. *Enhanced Emergency Response:*
- Ease of Use: The nasal spray’s user-friendly design allowsnasal spray2024 for quick and straightforward administration. The ease of use could make it more accessible for individuals who may struggle with injections or require assistance.
- Widespread Availability: The nasal spray could increase the availability of emergency treatment for anaphylaxis, particularly in locations where auto-injectors are not readily accessible. This could improve outcomes for individuals who experience severe allergic reactions outside of medical settings.
**2. *Potential Challenges:*
- Training and Education: Despite its ease of use, proper training and education on the nasal spray’s administration will still be essential. Patients and caregivers need to understand when and how to use the spray effectively, as well as recognize the symptoms of anaphylaxis.nasal spray2024
- Cost and Insurance: The cost of the nasal spray and its coverage by insurance will be critical factors in its adoption. Ensuring that the medication is affordable and accessible to those who need it will be important for maximizing its impact.
Broader Implications for Allergy Treatment
The approval of the nasal spray is part of a broader trend in advancing allergy treatment and improving patient outcomes.
**1. *Innovation in Allergy Treatment:*
- Emerging Therapies: The development of new treatment options, including nasal sprays and other innovative therapies, reflects ongoing progress in the field of allergy management. These advancements offer patients more choices and potentially better outcomes.nasal spray2024
- Personalized Medicine: Advances in allergy treatment also include efforts to personalize therapies based on individual needs and responses.
**2. *Ongoing Research and Development:*
- Future Innovations: Research continues to explore
Conclusion
The FDA’s approval of the first nasal spray for treating dangerous allergic reactions represents a significant advancement in allergy management. By offering a more accessible and user-friendly alternative to traditional epinephrine injections, the nasal spray has the potential to enhance emergency response and improve outcomes for individuals at risk of anaphylaxis.
As the nasal spray becomes available, education and training willnasal spray2024 be crucial in ensuring its effective use. The introduction of this new treatment option underscores the ongoing progress in the field of allergy care and highlights the importance of continued innovation and research.
With the potential to improve accessibility and ease of use, the nasal spray could become a valuable tool in managing severe allergic reactions and providing peace of mind to individuals and families affected by allergies.









